There was an invigorating sense of renewed energy surrounding the 13th annual DDF (Drug Delivery & Formulation) Summit in Berlin. And thank goodness for that. Global pharma is hitting a major fork in the road, and one way leads to failure. For me it’s vital that the industry comes together to grasp the once-in-a-generation opportunity that emergent technology is offering. If we act now, the promise of multidimensional, smartly connected, patient-centric care will be realised – but only if we collaborate more.
I’m under no illusions that my call for collaboration chimes better with the future than it does with the traditionally siloed R&D streams of pharma. But that’s entirely the point. The winners in the new technology landscape will be the companies that can adapt and integrate new approaches early and efficiently into their development plans. And that means coming to terms with how aspects such as engineering, digital health and innovative drug delivery devices can knit together to expand the boundaries of the druggable space.
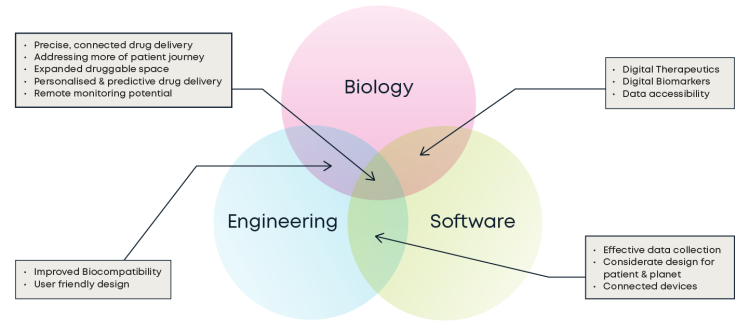
Let me start with engineering. I’m fortunate that my perspective has been broadened by recent experiences here at CC. Our commitment to biopharma has been crystalised into a bioinnovation capability that uniquely fuses expertise in biology, engineering and advanced computing to help deliver breakthrough products and services for clients. It’s been a real eye-opener and inspiration for me.
At DDF, the biology/engineering integration theme was seized upon during a lively panel discussion featuring experts from Janssen, Bayer, Lonza and Boehringer Ingelheim. Advances in AI, machine learning and low-power operation are enabling a new generation of smart, connected medical devices.
Interoperability of data for drug delivery
Ultimately, of course, the success of these devices depends on the quality and interoperability of data via integrated technology platforms, as deftly explained by my colleague Joe Corrigan, our Head of Intelligent Healthcare, who explains why digital biomarkers are vital for a patient-empowering connected health future. The panel at DDF unanimously agreed that the best results come when data scientists have a deep understanding of biology and the wider pharma landscape. The urgent imperative, then, is to close the loop between biological sciences and data.
For me, this is the way to hone drug delivery technology so that it offers truly powerful personalised and predictive elements. It’s vital to avoid a disconnect between what engineers conceive and what biology offers in terms of data, insight and intelligence. Attendees fielded plenty of questions around the technical feasibility and commercial opportunities of this kind of collaboration during a DDF speaker session on the importance of miniature active implants for drug delivery.
This topic flows nicely into the realm of Human Factors. Novartis used the DDF opportunity to reinforce the importance of implant and associated device design that constantly considers the why as well as the how. For me this is all about establishing what good looks like at the earliest possible stage.
However impressive and exciting it is, innovative technology will be compromised without a thorough and effective approach to HF studies. This is a multi-dimensional challenge incorporating all the aspects I’ve been talking about, as well as human behaviour and psychology of course. Collaboration and a new spirit of agility is vital to ensure HF practices evolve to keep pace with technology.
Nanotechnology, from concept to reality
There was a real buzz in Berlin around the well-attended Bayer talk on how nanotech is moving from concept to reality in pharma. Their experts expounded on the ‘zone of possibility’ opening up around formulations for direct-to-organ treatment. I believe that – once again – this is an area of innovation that demands collaboration and a unified approach as early as possible.
It means, perhaps, shifting the focus from formulation to exploring and asking questions from the drug delivery device perspective. If we can design effective direct-to-organ delivery devices in tandem with novel, nanotech-derived formulations, we will propel the industry towards much more effective therapeutic mechanisms.
Biocompatibility is pivotal here, as we venture into a hitherto unknown world of atomic, molecular and super-molecular scale research and development. Often, low compatibility between a device and a biological solution hinders efficacy. It’s absolutely crucial to understand how the key components interact with one another so that results are not adversely affected.
I think by now you’ll have digested the spirit of my reflections in the wake of DDF. The summit proved that the industry is moving at pace, leaving behind the traditional world of drug formulations and delivery. But we need to be doing more. My day-to-day work at CC is framed by a collective vision – a belief in a future unconstrained by current thinking – which has real resonance in this space.
Let’s challenge the status quo. Let’s make sure bold questions are asked at the concept stage. Let’s be willing to unify complementary disciplines and expertise for the common cause. An unprecedented and thrilling convergence of new technologies is happening right now – there’s never been a better time to exploit it so we can improve patient outcomes around the world. Drop me an email if you’d like to continue the conversation on the topic, I’d love to hear your perspective.





