Innovation in drug delivery devices has evolved at a constant pace for 30 years. From humble prefilled syringes to injection pens, autoinjectors, electromechanical injectors and on-body pumps, each marked a significant advance on their predecessors in flexibility of formulation, volume, patient comfort and sophistication when it comes to improving outcomes and avoiding mistakes while self-administering lifesaving drugs. As for what’s next, our belief is that we sit on the cusp of a decade of innovation and commercial opportunity in active implants.
This view is a result of our deliberations over a key question facing the industry – what will be the next big innovation area in drug delivery technologies? We took a deep dive into the evolving R&D pipeline of key therapeutic areas, including cardiovascular, the central nervous system (CNS), rare diseases and oncology. We concluded that drug delivery innovation will respond to a number of complex conditions requiring long-term – or lifelong – treatment to maintain, improve or cure the disease or condition. We identified oncology as the area most primed for revolution because of evolving treatment regimen and drug development.
As an evolving next-generation platform, active miniature implants are well-equipped for the coming challenge. They combine long-term drug administration with a range of disease-specific capabilities such as discrete administration, patient and caregiver safety, and smoother pharmacokinetics and pharmacodynamics profiles than the peaks and valleys of frequent injections.
In oncology, where survival is getting longer and prognosis is improving dramatically, many therapies require administration over many years or for life. The same is true in CNS, where drugs for conditions such as Alzheimer’s and Parkinson’s are under development or going through regulatory reviews. Difficult to treat conditions such as certain types of schizophrenia also require prolonged treatment.
Such trends combine with groundbreaking advances in electronics, silicon chips, microfabrication technologies, sensors and advanced materials to ensure that active implants will dominate the coming innovation cycle for drug delivery technologies.
With a unique breadth of multidisciplinary skills, Cambridge Consultants is well placed to advise clients on the many building blocks necessary for successful innovation. In this article, I’m going to draw on that expertise to provide a broad technical overview of the key components. I hope the insight will inform and inspire ambitious innovators looking to plot a path to success in this exciting space.
Let’s begin with a look at the architecture of a drug delivery system, as illustrated below. This implantable device is partially or completely introduced to the human body and stays in place after the procedure. It boosts effectiveness by controlling release rates, dose intervals, and the placement of drugs into the body. Other benefits can include closed loop control and communication of dose and therapy events, as well as remote monitoring and updates to treatment.
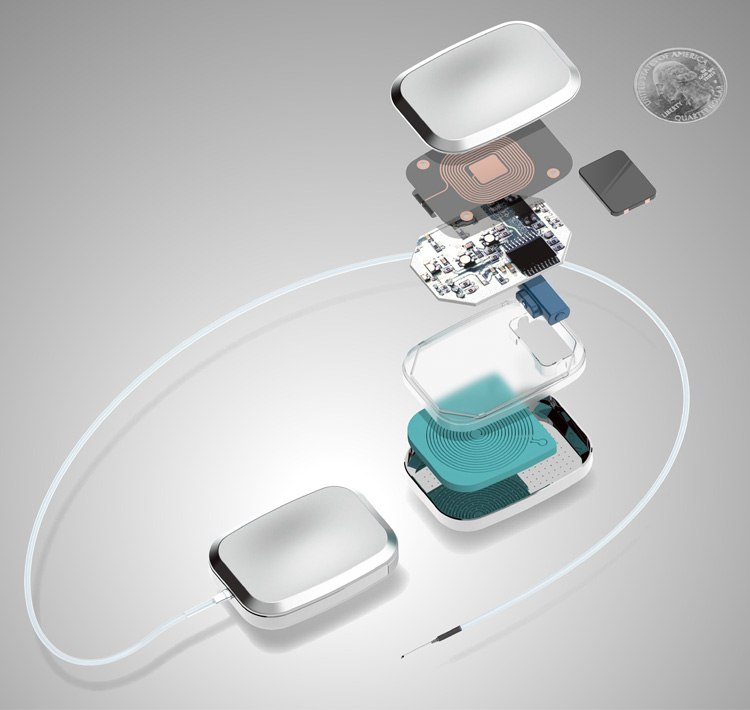
The options for drug delivery and release
Right now, plenty of innovation is focused on the crucial function of drug delivery and release. With many options available to find the appropriate method for a particular biologic, we think that a combination of active and passive technologies is the way forward.
Micropump systems are one of the more common delivery methods. They use a variety of pump technologies with different approaches for reservoir priming. These pumps force the drug from the implant body directly or through an implanted catheter into the targeted infusion site. The technologies often used are electrostatic, piezoelectric, electrochemical and thermal. However, there are newer, less used technologies that can be applied if low dosage and extreme miniaturization are required.
Microspouter technology consists of a combination of polymer casing containing a reservoir and a sponge that can be distorted using magnetic fields. While the focus has been in passive implant applications so far, this technology has the potential for active implants.
Nanochannel delivery systems use thousands of tiny tube-like structures to regulate the release of medicine. This technology is currently being tested in implantable capsules and could also be used for localized delivery. Combining it with an active implant has the potential for very precise control of drug levels with the ability to change delivery rates over time.
Osmotic pump technology uses an inner core that combines a drug with a layer of osmogens. The outer coating of the pump is made with a semi-permeable membrane allowing water to enter and expand the osmogen layer. This expansion compresses the internal reservoir which forces the drug out through a flow limiter for delivery at a set rate. While often used in passive implants, this technology could be used to design low power active implants consisting of multiple cells that provide extended therapy.
Drug storage technologies
Drug storage is closely coupled to delivery. The way a drug is stored within an implant is often driven by its physical characteristics. Trade-offs are made during the drug discovery process which prioritize stability and acceptability to the patient. In some cases, excipients can be added that improve viscosity, enhance solubility, increase absorption, or extend drug life while in vivo. Not all drug formulations can be adapted to have all the desired characteristics, so a variety of storage technologies are used to support integration into an implant.
Bulk reservoirs are often used for liquid formulations within the implant. At low volume this could last the life of the implant however, in many cases refills might be necessary. Where refilling a liquid drug is required, an implant can be engineered with a variety of technologies to help identify location of the implant and specifically the refill port even when in the body. Light, electrical signalling, magnetics, and even the physical shape of the implant can all be used to guide a needle and simplify the task of refilling.
Micro reservoirs often combine drug storage and drug delivery functions. The drug dispersal is divided into multiple chambers that when opened, diffuse into the body. The smaller reservoirs can contain liquid or solid drug formulations that degrade and release with time. These small wells of drug can make use of active or passive methods for promoting diffusion into the body. Micro reservoirs can be more costly to design than bulk storage methods but can often be made to fit an implant form factor better.
A broad range of implant communications
Implant communications rely on a large range of technologies. One of the more common options is wireless RF. Here, the shape, size and location of the implant can affect the robustness of a solution. Body tissues are lossy and will degrade antenna performance, but antennas can be designed for implants that account for this effect and can still send data several meters outside the body.
RFID and near field RF can also be scaled to fit the needs of even very small implants and can be appropriate where low data rates are required. A passive RFID chip can be paired with a coil for extremely low powered communications. For implant use an off the shelf reader or tag is not usually appropriate. A custom communication coil can be designed as a stand-alone coil, integrated into a housing, or buried in the layers of a PCB.
Inductive link is a conventional method of communicating with implants outside the body. It usually requires a small gap of a few centimeters. It suffers from low data transmission rates and can be susceptible to activation by external electrical fields.
Body channel communications use an electric field with either galvanic or capacitive coupling. These approaches require very close proximity or are confined to within the body. Their main benefit is that they don’t require an antenna, which aids miniaturization.
Energy storage, charging and harvesting
Energy storage, charging and harvesting have a direct impact on the capability and duration of the implant. Primary batteries have a higher energy density than secondary batteries and offer the best energy per unit of volume. If the application uses enough power to require a backup or secondary battery, then the design becomes a bit more complex due to additional safety concerns, system design constraints, and supporting circuitry.
Primary batteries are broken into chemistries that are matched to the rate of discharge for the application. Low discharge rate is considered microampere range and includes materials like lithium/iodine. Medium discharge rate is considered milliampere range and this is where most drug delivery implants fall. Some examples here are lithium/manganese dioxide and lithium/carbon monofluoride. Finally, high-rate primary batteries are designed to deliver pulses in the ampere range. They include lithium/silver vanadium oxide and are commonly used in implanted devices for cardio-defibrillation.
Secondary batteries are usually found in applications with high power usage than drug delivery implants like neuro-stim. They can be used in a drug delivery implant if long-term or frequent dosing is required. Lithium-ion batteries are the most used as a secondary battery. There are safety concerns with these batteries regarding overcharge, short circuit, and the potential to rupture. Careful assessment of system and implant design is required to appropriately mitigate the risks that come with implanting lithium Ion technology.
Charging technology can also be used to extend the duration of an implant’s life. Inductive charging can be very effective. The biggest challenge is keeping the implant aligned with the external charging device comfortably, accurately, and for the duration of charge. Energy harvesting is not very common due to the difficulty of miniaturization and the characteristics of the energy produced. Simply integrating a larger battery is a much better fit for most applications.
The challenges of circuit board design
Circuit board design and electronic component selection can be more difficult for implants than for an application that does not need to be hermetic or biocompatible. While traditional PCB technology can be used, often when miniaturizing an implant, the circuit board becomes a structural piece of the design and part of the housing helping to seal in components that may not be biocompatible. Ceramic PCB technology can be made hermetic and can be used with brazing and welding to produce a sealed enclosure. Co-fired, thick film, and thin film ceramic technologies are all ceramic PCB options with different price points, limitations, and cost.
Co-fired ceramic is one monolithic piece that is built from layers of individual ceramic that have been printed or filled with metal structures. This material can be made to include passive components and can even be built up to make a complex 3D shape. It has the most flexibility but higher costs in both development and mass production. Thick film ceramic starts with a ceramic substrate but then uses a technique much like screen printing to additively apply alternating layers of resistive and dielectric material. Traces can be built up to have wells or pockets, but the geometry is much more constrained than co-fired boards. Thin film ceramic can provide high precision and extreme connection densities. Metal deposition methods and vacuum processing technology from semiconductors have been adapted to produce these boards. These boards are usually selected when co-fire or thick film cannot provide the performance needed and are typically the highest cost material.
Metal, ceramic or glass enclosures
Enclosure materials are heavily constrained by the need to be biocompatible and hermetic. The three main categories for long term implants are metals, ceramics, and glass.
Metals like titanium or stainless steel are relatively cheap and can work with laser cutting, stamping, or electron discharge machining. These materials resist corrosion, can withstand strain and repeated load stresses, and can be made lightweight. When integrating this material, careful design may be required to incorporate features like feed throughs as well as coexistance with RF communications.
Ceramics are biocompatible, stronger than glass, and do not block RF communications. Their main drawback is brittleness.
Glass is also RF agnostic but can be much more easily bonded. Low heat laser bonding is a common technique for assembly. The biggest downside is that it is the most brittle of the standard enclosure materials. Hence it is suitable for specific but limited applications depending up on the location and depth of the implant in the body.
So, to sum up, a broad understanding of the relevant technologies is vital in elevating a generic implant concept into a high-performance active device that will greatly enhance the efficacy of a treatment. They pave the way to unique benefits for patients who might struggle with traditional methods. Next-generation implants will improve patient experience, reduce the potential for toxicity and adverse effects, all while maintaining the high levels of adherence that is possible with injections and infusions. Please feel free to reach out to me to discuss any aspect of this topic. It would be great to continue the conversation one-to one.





