Client story
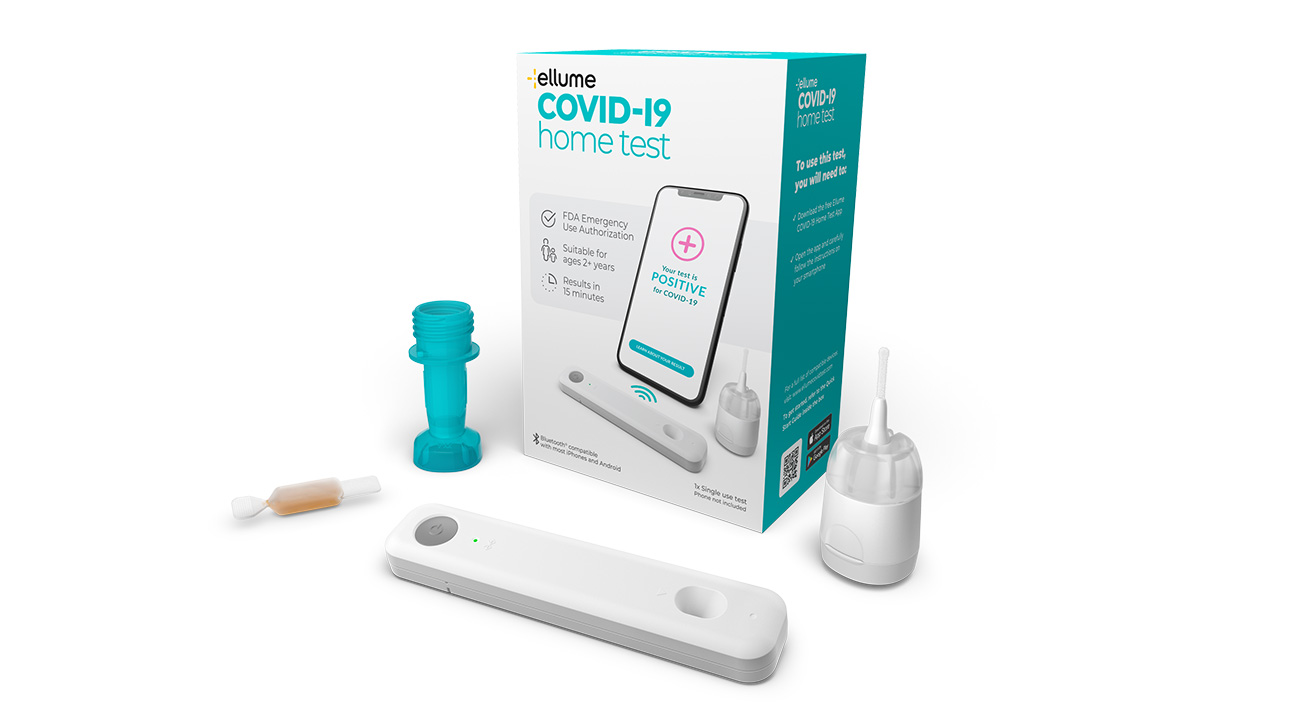
During one of the most critical periods of the global pandemic, Ellume’s over-the-counter, fully at-home COVID-19 diagnostic test became the first of its kind to be granted Emergency Use Authorisation by the US Food and Drug Administration (FDA). The news swept the world, because it opened the door to a new way of reducing the spread of the virus and help coax society back towards normality. The FDA authorised COVID-19 test remains a major milestone, made possible by ultra-sensitive optoelectronic detection technology from Cambridge Consultants.

Low-cost home-use technology
Controlling a spiralling, unpredictable pandemic demands mass testing that can’t be satisfied by a centralised programme alone. It must be moved closer to home. Ellume foresaw this acute need when company founder Dr Sean Parsons first came to us. With swine flu then threatening, Ellume initiated development of an at-home Rapid Flu Test (RFT). We were chosen as the key partner to help create low-cost, home-use technology – initially for influenza but ready for whatever was to come.
Ultra-sensitive optoelectronics
Close and enduring relationships were forged as our multidisciplinary global team drew on their broad expertise, including a deep understanding of lateral flow design. With Ellume taking the lead on assay development, the collaboration delivered on its objective: a versatile platform that is subsequently helping to fight the novel coronavirus through COVID-19 lateral flow testing.
Our pivotal sensing breakthrough is based on ultra-sensitive optoelectronic detection. For the COVID-19 test, it is coupled with Ellume’s supercharged fluorescent immunochromatography, using a quantum dot fluorescent particle, which delivers world-leading sensitivity and specificity.
The challenge

Low-cost mass manufacture
This is a story with a consistent theme: working alongside a client with the confidence and imagination to create a world-changing solution in the face of seemingly unsurmountable technical obstacles. Chief among the challenges was successfully balancing cost and performance. An at-home diagnostic must be very low cost and mass manufacturable. Yet hit-and-miss performance isn’t an option.
Each device that rolls off the production line must detect the target pathogen at low concentrations. The unique combination of biochemical assay and optoelectronic reader in the Ellume test achieves this. It also benefits from stringent attention to ease-of-use, vital for ensuring optimum performance for COVID-19 low-cost testing at home.

Label and reader in tandem
Lateral flow technology is well established as the basis for low-cost, home-use tests. Ellume decided to adopt the technology but to boost – considerably – its sensitivity. Most existing tests use labels to absorb light which present as coloured lines. Instead, Ellume opted to employ a highly efficient fluorescent label that would, when excited, emit light.
This created a new challenge for us. We had to develop a low-cost, high-performance fluorescent reader, matched to the chosen label and compact enough to integrate successfully.
“Richard Hall and his team of geniuses (in the truest sense) figured out the electronic/optical configuration that is the core of our detection technology. Coupled with the fluorescent nanoparticle (supernova) it’s what makes the technology sing. Without the brilliance of CC in the early days, we certainly wouldn’t have accomplished what’s happened.”

Vital signal chain analysis
A system perspective is vital to success. In a diagnostic such as this, it is the signal chain that demands most attention. What is the nature of the sample, how will it be transported by the strip, how will it interact with the conjugate pad?
How efficiently, together with its fluorescent label, will the target pathogen bind to the test line? What are the sources of background noise and what is the minimum concentration of the pathogen at the input that will be detectable at the output?
This was the level of tireless analysis that provided the answers we needed to clear the path to successful COVID-19 lateral flow testing.

Miniaturising the fluorimeter
Most fluorimeters rely on a set of optical and optoelectronic components – which we could neither afford nor fit into the device. The task was to find a way forward by reducing the number of parts, their size and their cost – without compromising performance.
Our solution implements the optics as two easily assembled plastic parts with no use of glass at all. These moulded plastic parts couple light between the test zones on the test strip and three optoelectronic components on the associated PCB.
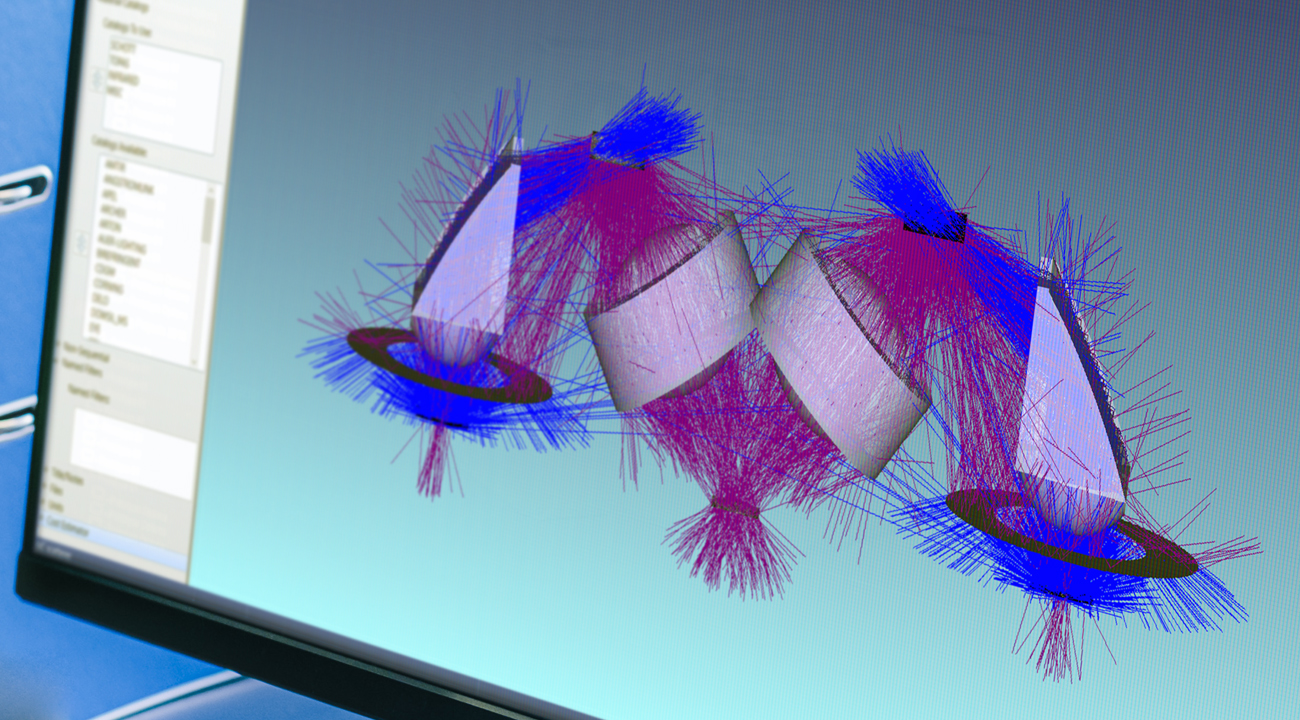
Precision optics but plastic parts
The two optical components are highly functional, tiny, low-cost plastic mouldings – and the end result of a complex design process. First the underlying optical concept, then exhaustive design by ray trace. After that, mechanical CAD was used to create parts that can be handled, assembled and moulded.
Material selection and supplier engagement were both conducted in parallel. We also worked out how to manage injection points, mould-flow, shrinkage and surface finish so that the critical optical functions can be maintained in mass-manufacturable parts.

Just enough electronics
The same theme permeates the electronics… how to achieve the required function at the lowest cost with minimal componentry. This boils down to the simplest possible microcontroller, a scattering of discrete components and a low-cost coin-cell battery. But relentless control of cost brings its own challenges.
The microcontroller has limited memory and the embedded code must be highly efficient. The coin cell battery offers only a low voltage and modest capacity. We had to respond with the shrewdest of approaches to power management.

FDA authorised COVID-19 test with 96% accuracy
As an over-the-counter, fully at-home FDA authorised COVID-19 test, the Ellume innovation is a world first. In December 2020 it was granted Emergency Use Authorisation by the FDA for non-prescription home use by adults and children aged two and above, with or without symptoms.
During a multi-site US clinical study, the test demonstrated 96% accuracy. The journey we set out on just a few years ago has helped to deliver an essential tool to help fight the spread of COVID-19. The whole Cambridge Consultants team is proud to be a part of it all.

A platform for the future
When it comes to world impact, Ellume’s platform technology has implications that extend beyond COVID-19 low-cost testing. Before the pandemic struck, the company partnered with QIAGEN N.V. to develop a diagnostic assay for tuberculosis.
This global scourge claims the lives of more than a million people annually and is the leading cause of death from a single infectious agent.